The World Bank may help indemnify Ebola vaccine makers in case any liabilities arise, as countries and international aid groups support drugmakers racing to develop preventions for the virus.
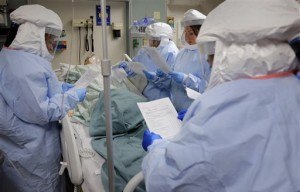
Establishing an indemnity mechanism is being discussed among governments, and the World Bank may play a role, said Marie-Paule Kieny, the World Health Organization’s assistant director-general of health systems and innovation.
Safety testing of experimental vaccines from GlaxoSmithKline Plc and NewLink Genetics Corp. are under way, with the next phase of trials to start by January. Protecting the manufacturers from liability is crucial as the clinical trials may not pick up some side effects, said David Heymann, a professor of infectious diseases at the London School of Hygiene and Tropical Medicine.
“The fear with Ebola trials is that if there is an adverse reaction, and it’s rare, that it won’t show up during the clinical trials,” Heymann, a former assistant director-general at the WHO, said in a phone interview. That could lead to a situation where “the vaccine will be considered safe and major populations will be vaccinated and these rare events would show up.”
Indemnification has been provided for previous vaccination campaigns—most notably for the 1976 swine flu epidemic, where manufacturers asked the U.S. federal government for protection against potential claims. While cases of Guillain-Barre Syndrome, a rare disorder that causes muscle weakness and sometimes paralysis, among vaccine recipients led to a “press frenzy,” the exact reason for the tiny increase in risk remains unknown, according to the U.S. Centers for Disease Control and Prevention.
Gulf War
Compensation from the U.S. government is also available to family members of Gulf War veterans who die from medically unexplained illnesses or infectious diseases.
World Bank spokeswoman Melanie Mayhew declined to comment on indemnification efforts for Ebola vaccines.
“We welcome the fact that this issue is being discussed,” said Catherine Hartley, a Glaxo spokeswoman in London. “Indemnification provisions are an important part of the response to the Ebola epidemic.”
Johnson & Johnson, based in New Brunswick, N.J., and Novavax Inc., based in Rockville, Md., are also working on Ebola vaccines.
The World Bank is already mobilizing nearly $1 billion in financing for the countries hardest hit by the Ebola crisis. This includes more than $500 million for the emergency response and to help speed up the deployment of foreign health workers and at least $450 million from the IFC, a member of the World Bank Group, to enable trade, investment and employment in Liberia, Guinea and Sierra Leone, the most-affected countries.
Treatments Sought
Clinical trials for Ebola treatments will begin alongside vaccine testing next month, and the medical aid charity Doctors Without Borders has asked each of the research units running them to ensure they have indemnity insurance. The University of Oxford will provide indemnity cover for a trial to test Chimerix Inc.’s antiviral brincidofovir.
In the search for vaccines, different types of studies will be done in the three West African countries.
The U.S. National Institutes of Health plans to run tests in Liberia using a classical randomized placebo-controlled trial design, in which about 9,000 people would get the vaccine and another 9,000 would get a placebo, Kieny said. Researchers will monitor the number of Ebola infections in each group to see whether the vaccines afford any protection.
In Sierra Leone, the trial will be a so-called stepped-wedge study in which no placebo is used and volunteers are added gradually and results accumulated over time, she said.
In Guinea, the trial design is yet to be decided, but the WHO is in discussion with researchers from Norway, France and Canada about a so-called ring vaccination design in which shots would be given to people in communities where there are infections to see if further transmission can be prevented, Kieny said.
(AP Photo/Eric Gay, File)





















 Modern Underwriting Technology: Decisive Steps to Successful Implementation
Modern Underwriting Technology: Decisive Steps to Successful Implementation  Winter Storm Fern to Cost $4B to $6.7B in Insured Losses: KCC, Verisk
Winter Storm Fern to Cost $4B to $6.7B in Insured Losses: KCC, Verisk  Lessons From 25 Years Leading Accident & Health at Crum & Forster
Lessons From 25 Years Leading Accident & Health at Crum & Forster  Execs, Risk Experts on Edge: Geopolitical Risks Top ‘Turbulent’ Outlook
Execs, Risk Experts on Edge: Geopolitical Risks Top ‘Turbulent’ Outlook 



